Recently, the research on the "electrocatalytic conversion of carbon dioxide" by the Chinese Academy of Sciences Shanghai Institute of Technology has progressed. The relevant results were published in the international journal "German Applied Chemistry". Through electrocatalytic CO2 conversion, renewable wind power, solar power and other clean electrical energy are used as energy sources to convert CO2 directly into carbon monoxide, formic acid and other fuels and chemicals under normal temperature and pressure conditions, and at the same time realize the utilization of carbon dioxide resources and cleanliness. Effective storage of electrical energy. It is reported that after nearly two years of exploration, the Chinese Academy of Sciences found that Pd-Sn alloy catalysts composed of metallic palladium (Pd) and tin (Sn) have very excellent properties. With only a very low voltage applied, 99% of the input power (current efficiency) can be used to drive carbon dioxide conversion to formic acid. Formic acid is one of the basic organic chemical raw materials and is widely used in industries such as pesticides, leather, dyes, medicine and rubber. In addition, the team also developed nitrogen-doped mesoporous carbon (N-carbon) materials for electrocatalytic carbon dioxide conversion. By regulating the pore structure and surface active site configuration of N-carbon, the direct conversion of carbon dioxide to ethanol was successfully achieved. Ethanol is one of the most widely used basic chemicals used in the synthesis of acetic acid, beverages, flavors, dyes, and fuels. Biological Chemical Series,Biological Reagent Organisms,Biological Reagent Reaction,Sodium Dodecyl Sulfate K12 XINGZHILIAN BIOLOGICALR&D CO.,LTD , https://www.xzlsdslds.com
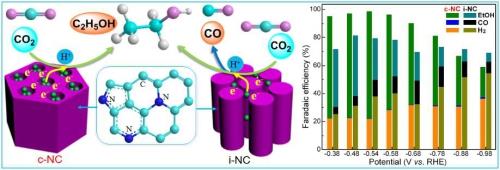
Electrocatalytic Oxidation of Carbon Dioxide to Ethanol Based on Nitrogen-containing Mesoporous Carbon