The efficient and clean use of fossil fuels is of great significance for solving increasingly serious environmental problems. Nitrogen oxides (NOx) produced during combustion can cause environmental problems such as acid rain and photochemical smog. Therefore, it is necessary to strictly control NOx emissions. At present, NOx emissions mainly come from the combustion process of nitrogen-containing fuels. By establishing the combustion dynamics model of nitrogen-containing fuel, it is possible to grasp the law of nitrogen transfer during the combustion of nitrogen-containing fuel, which has important guiding significance for controlling NOx emissions. Recently, researchers in the New Technology Laboratory of the Institute of Engineering Thermophysics, Chinese Academy of Sciences have conducted relevant studies on the low-temperature oxidation and pyrolysis processes of nitrogen-containing fuels, using tunable vacuum ultraviolet photoionization and molecular beam mass spectrometry to study the nitrogen-containing fuels. Model molecules, nitromethane and pyridine pyrolysis and low temperature oxidation Main products and intermediate components, the experiment found new intermediate products, based on the experimental development of nitromethane and pyridine kinetic models. The formation rate analysis shows that during the low-temperature oxidation of nitromethane, due to the carbon-nitrogen bond fission and roaming-mediated isomerization, the main decomposition pathways of nitromethane pyrolysis and oxidation are different; while in the low-temperature oxidation of pyridine, Pyridine is mainly consumed through C5H5N → C5H4N → C5H4NO2 → HCN + CO + CH2CHCO and C5H5N → C5H5NO → C2H2 + HCN + CH2CO. Sensitivity analysis shows that carbon-nitrogen bond breaking and roaming isomerization promote the consumption of nitromethane during low-temperature oxidation and pyrolysis; in the low-temperature oxidation of pyridine, C5H4N + O2 => C5H4NO2 and C5H5N + The OH = C5H4N + H2O reaction has a significant promotion effect on pyridine consumption, while C5H4N + HO2 = C5H4NO + OH has a strong inhibitory effect. The above research is supported by the National Natural Science Foundation of China's major research program, the General Fund, the key research and development plan of the Ministry of Science and Technology and the Youth Thousand Talents Program of the Central Organization Department. Related research published two papers in Combustion and Flame (2019, 202: 394-404; 2019, 203: 247-254). Ningbo Kyson Cool Electronic Technology Co., Ltd. , https://www.kysoncool.com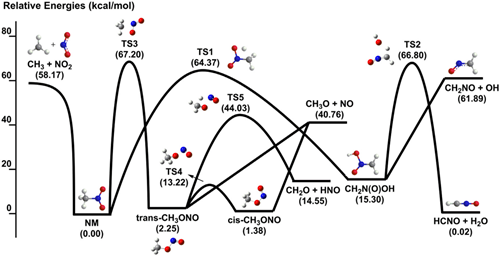
Fig.1 Potential energy surface of nitromethane decomposition at 0K 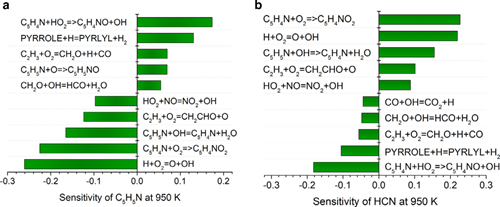
Figure 2 Sensitivity analysis of (a) pyridine and (b) hydrogen cyanide at 950K