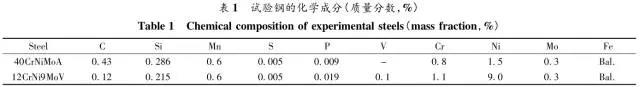
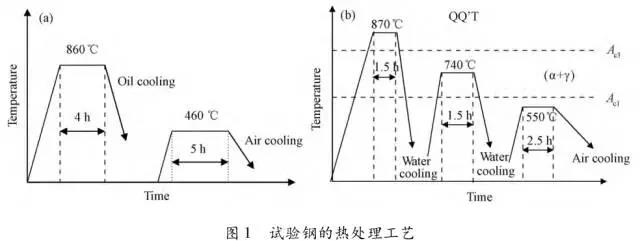
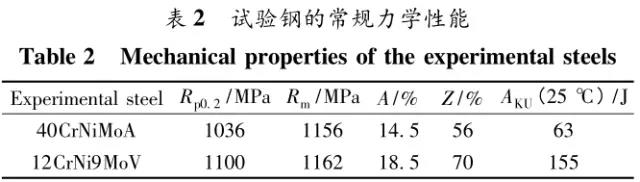
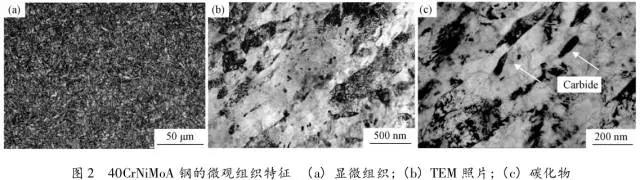
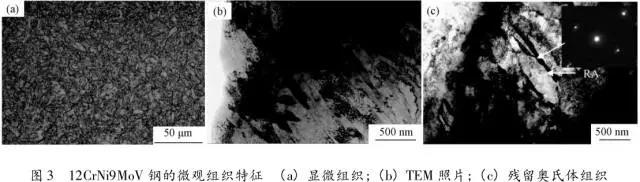
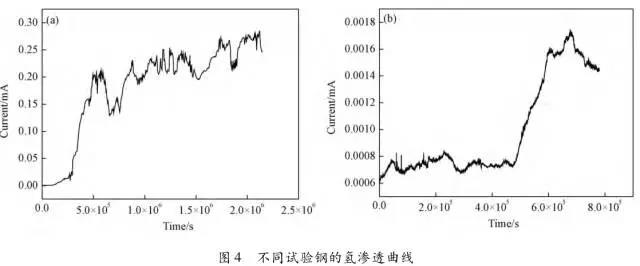
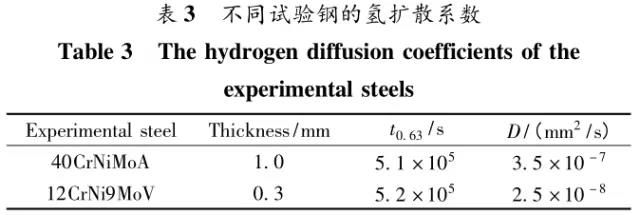
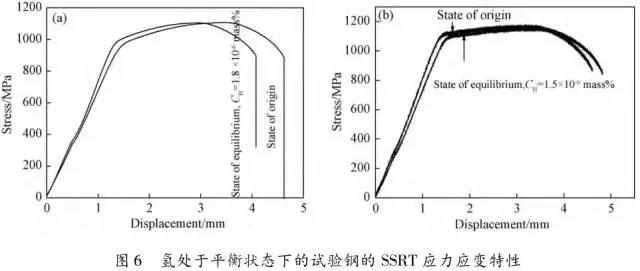
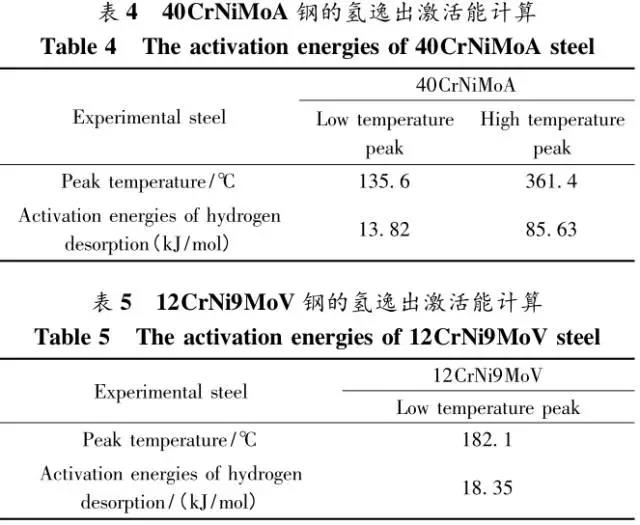
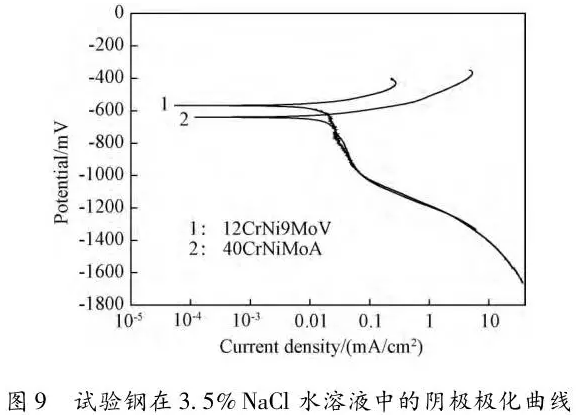
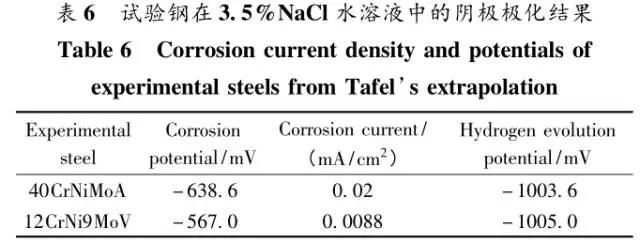
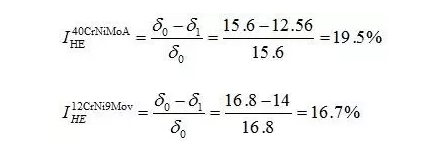Through the heat treatment of quenching + secondary quenching + tempering (QQ ' T) process for 12CrNi9MoV steel with high strength bolts , the diffusion and trap behavior of hydrogen and the hydrogen embrittlement sensitivity of the steel were studied. Steel 40CrNiMoA was compared. The results showed that low-carbon steel, high nickel 12CrNi9MoV through QQ 'T has a good overall performance; compared to the same strength level 40CrNiMoA steel, having a lower diffusion coefficient of hydrogen in 12CrNi9MoV; the ability to hydrogen-induced fracture resistance of the steel 12CrNi9MoV Stronger, the type of hydrogen traps present in the steel are dislocations and grain boundaries; and in the seawater environment, the hydrogen evolution tendency of 12CrNi9MoV steel is weaker. Cr-Ni-Mo-V alloy steel is widely used due to its high strength and good plastic toughness, especially as a steel for bolts. Its mechanical properties and corrosion resistance are better in harsh and complex marine environments. A large number of marine engineering equipment has been promoted and used. The general engineering structure steel 40CrNiMoA has high strength and good toughness matching. When the tensile strength is less than 1000 MPa , it is often used to manufacture large-size bolts with good performance. However, as the strength level of the bolt increases, the hydrogen embrittlement sensitivity of the material increases, posing a safety hazard to the normal operation of the equipment. Adding V element to Cr-Ni-Mo steel, increasing Ni content and reducing carbon content, through quenching and tempering treatment, not only can maintain high strength of steel, but also can significantly reduce the hydrogen embrittlement sensitivity of materials, which is very important engineering. significance. The high-strength bolt steel 12CrNi9MoV is taken as the research object, and the 40CrNiMoA steel is the comparison object, in order to provide theoretical and data support for the rational selection of high-strength bolts for marine engineering. 1 Experimental materials and methods The 12CrNi9MoV steel used in the experiment was smelted into an electrode by an intermediate frequency furnace, and the electroslag was remelted into a steel ingot, and finally forged into a 50 mm bar. The 40CrNiMo steel was vacuum inductively smelted and cast into an electrode, and then remelted to obtain an ingot by electroslag remelting. It is also forged and rolled into a bar after forging. The chemical composition of the two test steels is shown in Table 1 . The heat treatment processes of 40CrNiMoA and 12CrNi9MoV steel are quenching + tempering (QT) and quenching + secondary quenching + tempering (QQ ' T) respectively . The specific process is shown in Figure 1 . The microstructures of the 40CrNiMoA and 12CrNi9MoV steels after heat treatment were observed under the Leica DMI 500M optical microscope; the micro-areas were observed on the PHILIPS CM200 transmission electron microscope; the conventional mechanical properties were tested on the SINTECH20/G tensile tester; Slow strain rate test ( SS RT) was performed on a WLDM-100 slow strain rate stress corrosion tester; hydrogen diffusion coefficient and polarization curve were respectively performed in an electrochemical test device Gill AC Bi-STAT electrochemical workstation. Etc. Determination of the trap behavior of hydrogen in steel on the temperature-desulfurization analysis equipment; the hydrogen content in the sample is obtained by cathode hydrogen charging method, and the hydrogen charging equipment is DH1715A-5 DC double-tracking regulated steady current power supply. . 2 Experimental results and analysis 2.1 Mechanical properties of test steel The mechanical properties of the test steel after heat treatment are shown in Table 2. It can be seen from the table that the mechanical properties of the two test steels are good, especially for 12CrNi9MoV steel, which has excellent toughness. 2.2 Microscopic observation Figure 2 shows the microstructure of 40CrNiMoA steel. It can be seen from the figure that after heat treatment of 40CrNiMoA , the microstructure of the 40CrNiMoA exhibits a typical tempered sorbite structure, as shown in Fig. 2 (a) . According to the transmission electron microscope observation of 40CrNiMoA steel, it is known that there are many carbides in the steel, and the presence of these carbides easily becomes a strong hydrogen trap, making it difficult for hydrogen atoms to escape from the matrix. The microstructure of 12CrNi9MoV steel after heat treatment is shown in Figure 3 . It can be seen from the figure that after quenching by QQ ' T , the steel exhibits a typical tempered sorbite structure. It can be seen from transmission electron microscopy that there is residual austenite ( RA ) in the steel, and there are few carbide particles (Carbide) . 2.3 Hydrogen diffusion capacity in test steel In order to characterize the diffusion ability of hydrogen in different test steels, the hydrogen diffusion coefficient of 40CrNiMoA and 12CrNi9MoV steels was studied and determined by hydrogen permeation test method. At the same time, the determination of the hydrogen diffusion coefficient provides data support for studying the hydrogen accumulation at the stress concentration of high-strength bolts and reaching equilibrium. (a) 40CrNiMoA (b) 12CrNi9MoV According to the hydrogen permeation curve, the hydrogen diffusion coefficients of different test steels were obtained by time lag method, as shown in Table 3. In the table, t 0. 63 is the lag time, and D is the apparent hydrogen diffusion coefficient obtained by the time lag method. It can be seen from Table 3 that the hydrogen diffusion coefficient of 40CrNiMoA steel is significantly larger than that of 12CrNi9MoV steel, which is due to the microstructure of the two steels, the crystal structure, the number and type of inclusions in the steel, the second phase and the dislocations formed by plastic deformation. closely related. Residual austenite exists in 12CrNi9MoV steel, which blocks the diffusion of hydrogen. In 40CrNiMoA steel, there are carbides, inclusions, etc., such as inclusions MnS , and its orientation in steel is also an important factor affecting the hydrogen diffusion coefficient. When the strip-shaped MnS inclusion is in the same direction as the hydrogen diffusion, the inclusion is a hydrogen diffusion channel; when its orientation is perpendicular to the hydrogen diffusion direction, the diffusion of hydrogen is hindered. Thus, the difference in the microstructure of the test steel resulted in a change in the hydrogen diffusion behavior of the two steels. In engineering applications, the hydrogen diffusion coefficient can be used to determine the hydrogen concentration of the high-strength bolt surface under hydrogen evolution conditions, and the theoretical concentration of hydrogen at a certain position in the steel. 2.4 Stress-strain characteristics and plasticity of test steel under different hydrogen contents The slow strain rate tensile test of a smooth sample under hydrogen charging conditions can reflect the effect of hydrogen on the stress-strain properties and plastic loss of the material. For high-strength bolts, plasticity can play a role in relieving the stress concentration at the root of the notch, while the reduction in area can better reflect the effect of hydrogen on the properties of the material, which is more sensitive to tissue changes. Therefore, studying the stress-strain characteristics and plasticity of test steel under different hydrogen contents is of great significance for hydrogen-induced delayed fracture of cognitive high-strength bolts. Since the stress-strain curves of different steel grades have no contrast with the change of hydrogen content, this section selects 12CrNi9MoV steel as the research object, in order to obtain the influence of the change of hydrogen content on the stress-strain characteristics of steel. Figure 5 shows the stress-strain characteristics and plasticity of 12CrNi9MoV steel under different hydrogen contents. It can be seen from the figure that under different hydrogen charging time, the SS RT stress-strain curves of the test steel are close to each other, and the intensity changes are less, indicating that the influence of hydrogen on the strength of the steel is small, as shown in Fig. 5(a). The plasticity of the test steel at different hydrogen contents is shown in Figure 5(b) . The elongation after fracture and the reduction of the area with the increase of hydrogen content are not obvious, which indicates that under a certain hydrogen content, the steel can still relieve the stress concentration by its good plasticity, thus prolonging the time of hydrogen induced delayed fracture. . 2.5 Bearing capacity of hydrogen in equilibrium in test steel According to Juliet, there is a concentration gradient from the surface to the core after the cathode is hydrogen-charged. Hydrogen equilibrium state in the material shall be defined as: a sample placed in the atmosphere of hydrogen content after several days in a stable state. In order to evaluate the mechanical properties of hydrogen under equilibrium conditions in different types of high-strength bolt steel under actual working conditions, the samples under the same cathode hydrogen-filling conditions were saturated and hydrogen-charged, and then placed in room temperature air for 340 h, according to the study. As a result, it can be seen that the hydrogen content at this time is at a stable value, and the state of the sample is defined as the application state in which the hydrogen is in a saturated state under actual working conditions, and the hydrogen embrittlement sensitivity of the steel for different bolts in this state is evaluated. Figure 6 shows the SS RT stress-strain characteristics of 40CrNiMoA and 12CrNi9MoV steels with hydrogen in equilibrium. The hydrogen content of the 40CrNiMoA and 12CrNi9MoV steels after hydrogenation was 2.8 h , and the equilibrium hydrogen contents were 1. 8 × 10 - 6 and 1.5 × 10 - 6 ( mass fraction, the same below ) . It can be seen from the figure that the equilibrium hydrogen content has little effect on the strength of the two test steels, and the elongation has a significant downward trend. It is calculated that the elongation of the 40CrNiMoA steel in the equilibrium state of the hydrogen and the uncharged state decreased from 15.6% to 12.56 % , and that of the 12CrNi9MoV steel decreased from 16.8 % to 14% . The hydrogen embrittlement sensitivity of the test steel is represented by I HE . The larger the value, the higher the sensitivity. The expression is : I HE =(δ 0 -δ 1 )/δ 0 × 100% Where: δ 0 is the elongation of the sample when not hydrogenated; δ 1 is the elongation of the sample after hydrogen charging. (a) 40CrNiMoA (b) 12CrNi9MoV It is calculated that the hydrogen embrittlement sensitivities of 40CrNiMoA and 12CrNi9MoV steels are :           Seen from the above results, 40CrNiMoA hydrogen embrittlement sensitivity of the steel is about 1.2 times 12CrNi9MoV steel. In engineering applications, The risk of hydrogen induced delayed fracture of 40CrNiMoA steel is higher, that is, the steel itself has weaker resistance to hydrogen induced fracture than 12CrNi9MoV steel. The effect of hydrogen on the strength and ductility of the test materials is attributed to the diffusion and aggregation behavior of hydrogen in different materials. After the sample is hydrogenated by the cathode, hydrogen atoms are easily diffused and aggregated into defects in the steel, such as inclusions, microscopic defects, etc., thereby causing greater stress concentration. The generation of hydrogen-induced microcracks is related to the decrease of hydrogen-induced bonding force; when the hydrogen concentration increases, the microcracks are further excited, and the hydrogen atoms aggravate the crack propagation by increasing the dislocation motion of the crack tip. In addition, the enriched hydrogen atoms also reduce the surface energy of the new surface, making the fracture prone to occur. Thus, the test steel after hydrogenation is more sensitive to hydrogen induced fracture. For different test steels, the difference in diffusion capacity and hydrogen solubility in steel leads to differences in hydrogen embrittlement sensitivity. The mechanical properties of 40CrNiMoA and 12CrNi9MoV steels after hydrogen charging are also closely related to the trap behavior of hydrogen in two steels. 2. 6 Trap Behavior of Hydrogen in Test Steel In order to study the existence form of hydrogen in 40CrNiMoA and 12CrNi9MoV steel, namely the trap behavior of hydrogen in steel, the TDS spectrum of hydrogen-filled test steel was studied by thermal desorption spectroscopy ( TDS) . The variation of the hydrogen evolution rate obtained by the study with the heating temperature and the hydrogen evolution activation energy are of great significance for understanding the hydrogen embrittlement sensitivity of different bolt steels. Figure 7 and Figure 8 show the TDS test results for 40CrNiMoA and 12CrNi9MoV steel, respectively. At different heating rates, the hydrogen evolution rate of 40CrNiMoA steel showed two peaks along the heating temperature. Hydrogen corresponding to a peak below 300 °C is generally referred to as diffusible hydrogen, and conversely non-diffusible hydrogen. It can be seen from Fig. 7(a) that there is non-diffusible hydrogen in the 40CrNiMoA steel, and the corresponding trap type is a strong hydrogen trap. The non-diffusible hydrogen content is less than the diffusible hydrogen. In general, the presence of a strong hydrogen trap can strongly restrict the escape of hydrogen atoms, making it difficult to diffuse in steel, but for high-strength bolts, the hydrogen in the strong hydrogen trap at the root of the thread is at a higher stress. Under concentration, diffusion and aggregation of microdomains occur. When the hydrogen content of the microdomain reaches a critical value, hydrogen-induced microcracks nucleate and expand, resulting in hydrogen-induced fracture. The TDS curve of the hydrogen-filled 12CrNi9MoV steel exhibits a single peak characteristic, and the peak temperature is below 300 °C, which is a weak hydrogen trap. When the steel is subjected to hydrogen diffusion treatment, the hydrogen atoms are easily escaped from the substrate, and the hydrogen removal is more thorough. By changing the heating rate, the relationship between the temperature and the heating rate of the two test steels was obtained, as shown in Fig. 7(b) and Fig. 8(b) , and the hydrogen evolution activation energy was obtained by linear fitting. Table 4 and Table 5 show the activation energies required for hydrogen atoms to escape from the matrix in the hydrogen-filled test steel at a heating rate of 100 °C / h . According to the corresponding relationship between trap activation energy and trap type, the hydrogen trap type in 40CrNiMoA steel is a weak hydrogen trap such as dislocation and lattice gap and a strong hydrogen trap such as carbide; the trap type in 12CrNi9MoV steel is mainly weak hydrogen trap. Dislocations, grain boundaries, etc. 2.7 Study on Electrochemical Characteristics of Test Steel In order to evaluate the corrosion behavior and hydrogen evolution characteristics of high-strength bolt steel 12CrNi9MoV, and to provide a basis for the rational selection of bolts, it is necessary to use the cathodic polarization test to study the difficulty of hydrogen evolution of the test steel in simulated seawater. Figure 9 and Table 6 show the cathodic polarization curves and Tafel treatment results of two test steels in a 3.5% NaCl aqueous solution, respectively. It can be seen from the figure that the cathodic polarization curves of the two test steels are first controlled by the activation of oxygen, to the diffusion control of oxygen, and then to the stage of hydrogen evolution control. According to the test results, in the seawater environment, compared with 40CrNiMoA (- 1003. 6 mV) , the hydrogen evolution potential of 12CrNi9MoV steel is lower, about -1005 mV , indicating that the steel has more ability to hydrogenate cathode in seawater environment. Weak, more suitable for the selection of low hydrogen brittle high strength bolts. 3 Conclusion 1) 12CrNi9MoV steel after quenching + secondary quenching + tempering heat treatment, its comprehensive performance is better, can meet the performance requirements of high-strength bolts; 2) For 40CrNiMoA and 12CrNi9MoV steels of the same strength grade, the diffusion ability of hydrogen in 12CrNi9MoV steel is weak; from the slow strain rate tensile test, 12CrNi9MoV has strong resistance to hydrogen induced fracture; 3) There are weak hydrogen traps such as carbide strong hydrogen traps and lattices, dislocations in 40CrNiMoA steel; weak hydrogen traps such as dislocations and grain boundaries exist in 12CrNi9MoV steel. In high-strength bolt applications, strong hydrogen traps have a detrimental effect on bolt safety; 4) In the seawater environment, 12CrNi9MoV steel has a weak tendency to cathodic hydrogen evolution. According to comprehensive analysis, low carbon high nickel steel is more suitable for the selection of low hydrogen brittle high strength bolts. Solar Led Garden Light,Solar Lamp For Garden,Solar Lamp For Landscape,Solar Lamp For Yard Shenzhen You&My Electronic Technology Co., Ltd , https://www.szyoumy.com